−Table of Contents
ClonalTools
Introduction
ClonalTools are a set of macros designed for the analysis of mosaic patterns. The macros correct for the effects of random clumping using the formula 1/(1-p) devised by Roach (1968) in the way outlined by West (1975, 1976) and West et al (1997).
Author
Dr Richard L. Mort MRC Human Genetics Unit MRC IGMM University of Edinburgh Western General Hospital Crewe Road Edinburgh. EH4 2XU, UK
Email: Richard.Mort@igmm.ed.ac.uk
All comments, criticisms, suggestions, feedback or bug reports would be gratefully received and appreciated.
Features
Action Buttons: Selecting ClonalTools from the toolsets dropdown menu installs 8 action buttons in the following order: circular selection, polygon selection, linear selection, batch mode, rotate right, rotate left, define neurite ROI and neurite distribution.
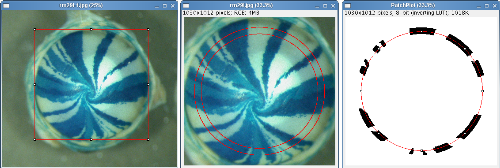
Circular Selection: The circular selection tool is optimised for the analysis of radial striping patterns of X-Gal stained X-inactivation mosaic eyes. Draw a square selection that touches the edge of the epithelium click the 'O' action button and select the scale to determine how far from the edge of the selection to sample. The default scale is 0.8 which samples the image using a circle 80% of the diameter of the corneal epithelium to avoid sampling too close to the limbal epithelium (Collinson et al 2002,. Mort et al 2009).
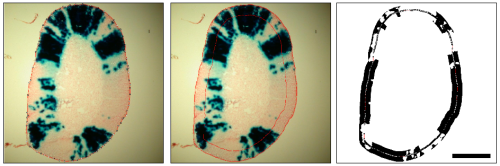
Polygon Selection: The polygonal selection tool is optimised for the analysis of radial striping patterns of X-Gal stained mosaic adrenal sections from the 21OH-lacZ mouse (Morley, S. D. et al 1996). This animal expresses β-Gal under the control of the 21-hydroxylase (21-OH) gene promoter directing expression in a tissue-specific manner to the adrenal cortex. The reason for the mosaic β-Gal expression in this mouse is unknown. Select the edge of the tissue using the polygon tool and then click the 'S' action button. The polygon selection is shrunk by an arbitrary 30 pixels to avoid sampling too close to the edge of the tissue.
Linear Selection: The linear selection tool is optimised for the analysis of mosaic patterns in X-Gal stained X-inactivation mosaic skin. When the 'I' action tool is clicked a line is drawn in the centre of a rectangular ROI parallel to its longest axis.
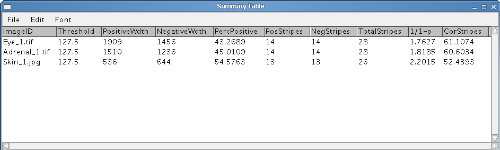
Summary Table: The 'Summary Table' logs all the information needed for a particular analysis. Everytime an analysis is run the results are added along with the image ID to the next empty row in this window. It should be noted that because in a linear selection the number of positive and negative stripes can differ separate values for 1/(1-p) and the corrected stripe number are calculated for the positive and negative cell type. For example, the corrected mean patch size could be calculated for patch sizes of only one cell population (e.g. only the positively-stained one or only the minority cell population) using the appropriate 1/(1-p) correction factor. This can provide a comparative index of the relative extent of cell mixing (West et al 1997).
Batch Mode: Using batch mode it is possible to analyse many pre-crpopped images (using the circular selection tool) and output the results. Just navigate to the folder of images you would like to analyse.
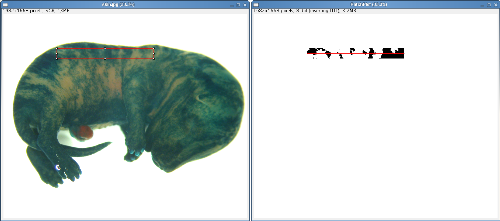
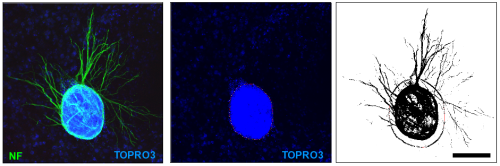
Explant Border: This tool displays the blue channel of the explant image allowing the researcher to trace the edge of the explant with the polygon tool.
Neurite Distribution: Having defined the explant border using the 'Explant Border' tool clicking the 'Neurite Distribution' button performs the analysis. The 'Neurite Summary Table' displays the percentage coverage of neurites in four regions of the image.
Description
Clonal Analysis of Mosaic Tissues The enzyme β-Galactosidase (β-Gal encoded by the LacZ gene) has been used widely for lineage marking in transgenic mice. When tissues expressing β-Gal are stained with the substrate X-Gal they appear blue whilst non-expressing tissues appear white. For example H253 X-inactivation LacZ mice express β-Gal in a mosaic pattern in all tissues due to random X-inactivation during development (Tan, S et al 1993).
Clonal Analysis Tools are designed to count and measure the number of β-Gal-positive and negative patches in digital images of tissue sections and whole samples from tissues exhibiting mosaic β-Gal expression patterns. Samples are analyzed by measuring along a circular, polygon or linear selection. The total number and total width of β-Gal positive and negative patches is determined and the number of corrected patches is calculated using the formula 1/(1-p) described by Roach in 'The Theory of Random Clumping' (Methuen 1968) where p is the proportion of the positive or negative cell type. For circular selections the corrected patch number is the same for both cell types, for linear selections the values may differ. This analysis corrects for the effects of variation in the proportions of the two cell types between samples. The corrected patch number assumes that the patch sizes are equal or normally distributed and that the positive and negative clones are randomly distributed throughout the tissue. The method has been used successfully for the analysis of stem cell function in the mouse corneal epithelium (Collinson et al, 2002, Mort et al 2009).
Analysis of neurite outgrowth in explant culture: Explant cultures of mouse retina develop radial outgrowths of neurites in patterns analogous to the radial stripes seen in X-Gal stained X-inactivation mosaic eyes. These patterns can be influenced by the genotype of the explant or the cells on which the explant is cultured (Pratt 2006, Tian et al 2008). ClonalTools includes a subset of tools developed to quantify such patterns.
References
Tan S-S, Williams EA, Tam PPL: X-chromosome inactivation occurs at different times in different tissues of the post-implantation mouse embryo. Nat Genet 1993, 3:170-174.
Roach SA. (1968). The theory of random clumping. London: Methuen.
Collinson, J. M., Morris, L., Reid, A. I., Ramaesh, T., Keighren, M. A., Flockhart, J. H., Hill, R. E., Tan, S.-S., Ramaesh, K., Dhillon, B. and West, J. D. (2002). Clonal analysis of patterns of growth, stem cell activity and cell movement during the development and maintenance of the murine corneal epithelium. Dev. Dyn. 224: 432-440.
Mort, R. L., Ramaesh, T. Kleinjan, D, A., Morley, S. D., and West. J. D. (2009). Mosaic analysis of stem cell function and wound healing in the mouse corneal epithelium. BMC Dev Bio 9:4.
West, J. D. (1975). A theoretical approach to the relation between patch size and clone size in chimaeric tissue. J. Theor. Biol. 50, 153-160.
West JD. (1976). Clonal development of the retinal epithelium in mouse chimaeras and X-inactivation mosaics. J Embryol Exp Morphol 35:445-461.
Morley SD, Viard I, Chung BC, Ikeda Y, Parker KL, Mullins JJ. (1996) Variegated expression of a mouse steroid 21-hydroxylase/beta- galactosidase transgene suggests centripetal migration of adrenocortical cells. Mol Endocrinol 10585:98.
West, J. D., Hodson, B. A., and Keighren, M. A. (1997). Quantitative and spatial information on the composition of chimaeric fetal mouse eyes from single histological sections. Develop Growth Differ 39, 305-317.
Morley SD, Chang SP, Tan SS, West JD. (2004). Validity of the 21-OH/LacZ transgenic mouse as a model for studying adrenocortical cell lineage. Endocr Res.(4):513-9.
Pratt, T. et al. (2006). Heparan sulphation patterns generated by specific heparan sulfotransferase enzymes direct distinct aspects of retinal axon guidance at the optic chiasm. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 26(26), 6911-6923.
Tian, N.M., Pratt, T. & Price, D.J. (2008). Foxg1 regulates retinal axon pathfinding by repressing an ipsilateral program in nasal retina and by causing optic chiasm cells to exert a net axonal growth-promoting activity. Development, 135(24), 4081-4089.
Installation
Clonal Analysis Tools relies on BinaryFilterReconstruct_.class written by Gabriel Landini. Install Gabriel Landini's Morphology Collection from http://www.dentistry.bham.ac.uk/landinig/software/software.html, download clonaltools_0.2a.txt.zip and extract to ImageJ/Macros/Toolsets folder.
Changelog
Version 0: 2009/03/19
Version 0.1: 2009/03/27
Added 'rotate left', 'rotate right', 'define neurite ROI' and 'neurite distribution' to toolset.
Version 0.2: 2009/04/21
Fixed bug that caused the circular analysis to stall.
Version 0.2a: 2009/05/13
Changed the neurite tools to be compatible with images stained with TOPRO3.
Version 0.2b: 2009/09/30
Fixed a bug that caused batch mode to stall after the first image.